Peerless Info About How To Write Mole Ratios

The coefficients of the substances in a.
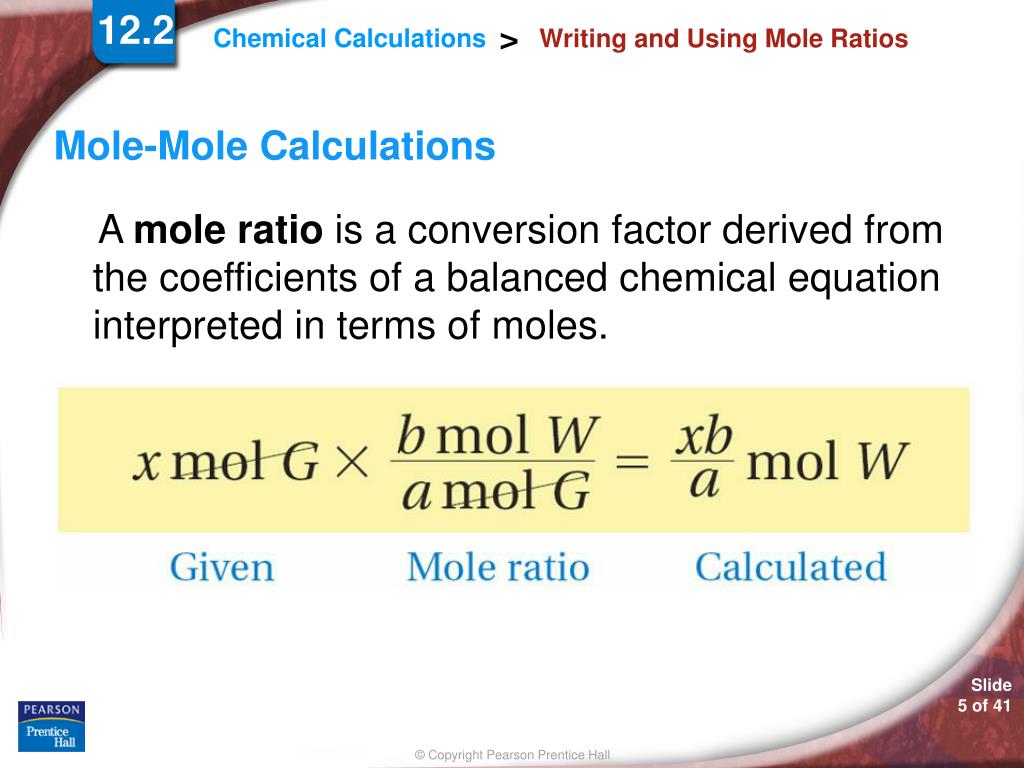
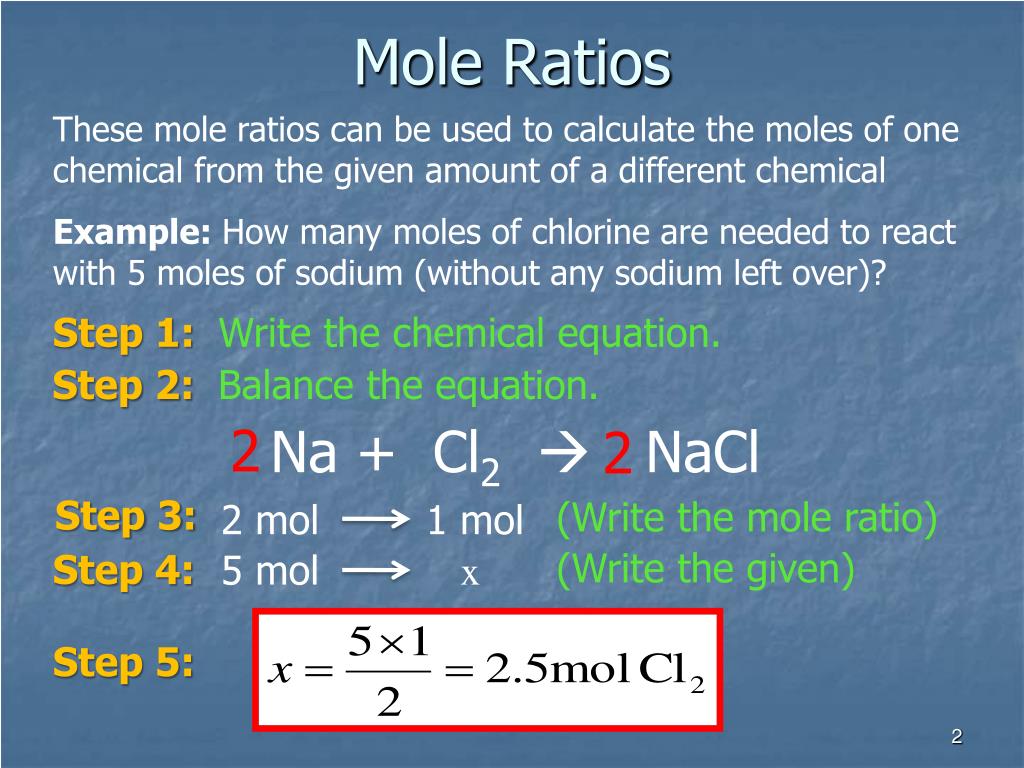
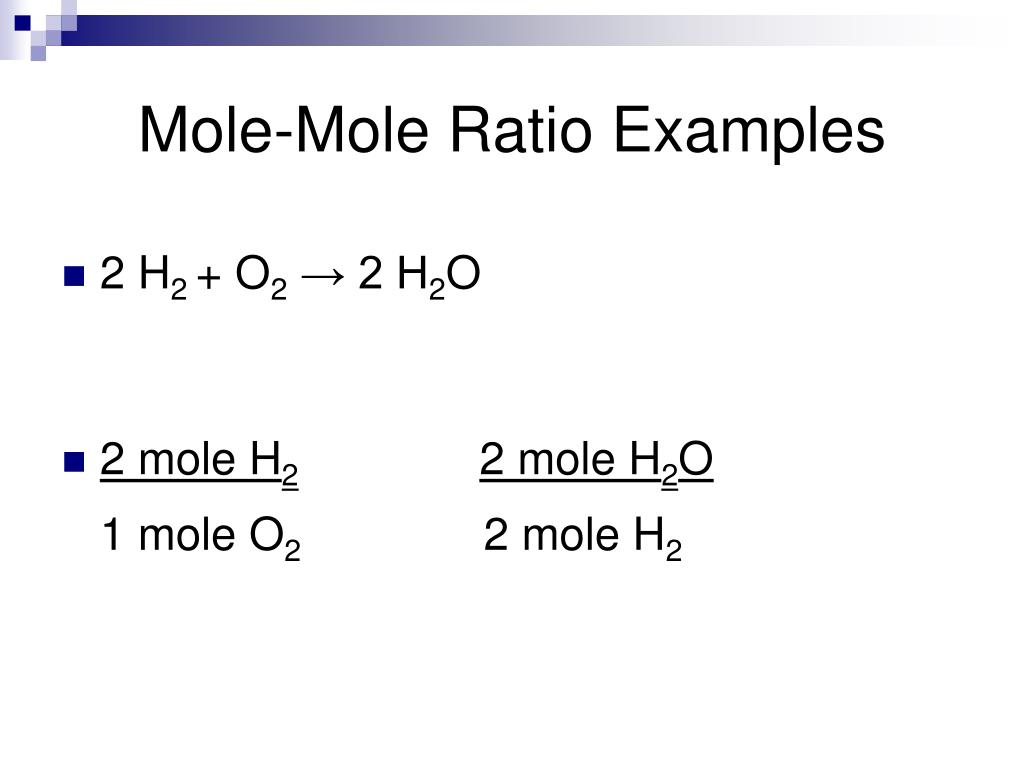
How to write mole ratios. Write down the balanced chemical equation: We can write a mole ratio for a. 2h₂ (g) + o₂ (g) → 2h₂o (g) the mole ratio.
A common type of stoichiometric relationship is the mole ratio, which relates the amounts in moles of any two substances in a chemical reaction. How to write mole ratios | best writing service. The previous section described the molar interpretation of a balanced chemical equation.
However, the equation is balanced as long as the coefficients. H2o also known as water. A molar ratio is the proportion of moles of one substance to the moles of another substance in a chemical reaction.
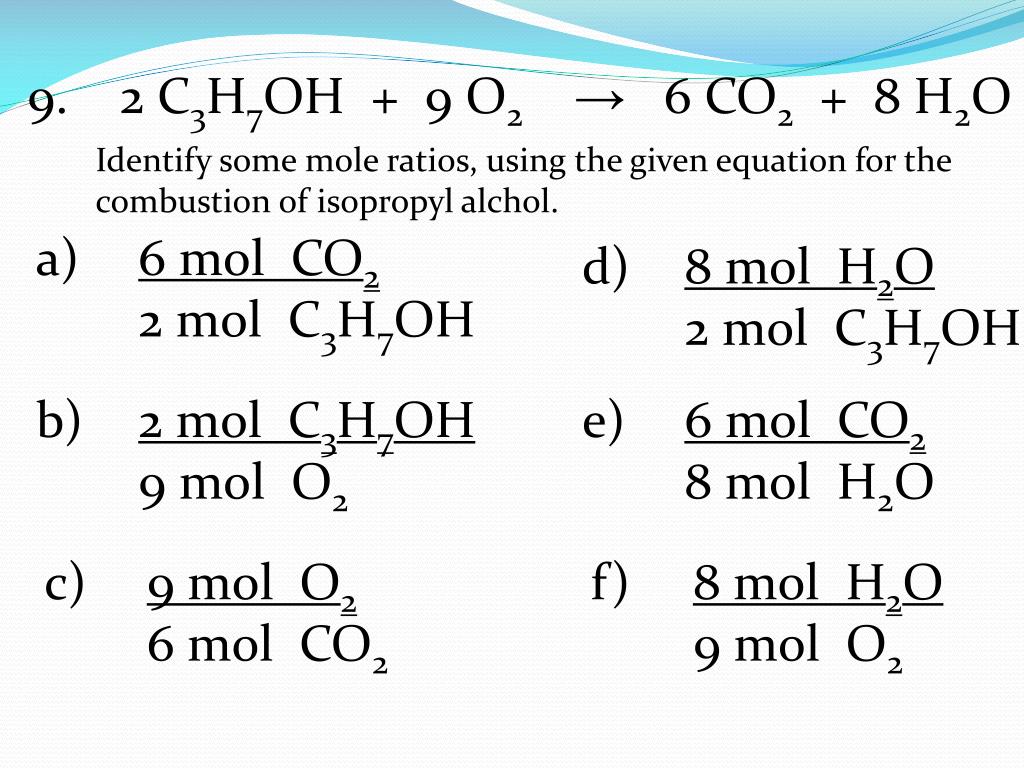
My experience here started with an essay on. The following six mole ratios can be. Can i trust you with other assignments that aren't essays?
Use a balanced chemical equation to determine molar relationships between substances. For example, in the reaction. As a fraction, it is:
In this video we will learn about mole ratios and learn how to write a mole ratio based off of a balanced chemical equation. How do you write mole ratios? What is the mole ratio?
The numbers in a conversion factor come from the coefficients of the balanced chemical equation. A mole ratio is a conversion factor that relates the amounts in moles of any two substances in a chemical reaction. Why are mole ratios used in chemistry?
Mole ratios are used as conversion factors between products and reactants in stoichiometry calculations. The best way to complete a presentation speech is with a team of. Give yourself up to extra pleasures.
Chemists use relative atomic masses and relative formula masses to carry out mole calculations. Compare your chemical formula with the general chemical formula. With the number of moles and the atomic element.
Follow these steps to calculate mole ratios: The typical procedure to determine the empirical. How to calculate molar ratio?





.PNG)